Miniaturization Makes Pneumatics the Choice in Medical Applications
From ambulance to operating room, recovery room to discharge at home, patients today encounter any number of medical devices that must be lightweight, small, safe, and often, portable. To maintain these safe, hygienic designs, most medical device manufacturers rely on pneumatics because they are highly customizable and compact.

As the demands for these types of components have evolved, so have the pneumatic cylinders and valves used in them. The need for smaller, lighter devices has translated into a push for valves that offer more flow at lower power (often battery power). In addition, this all means the need for lighter materials, so plastic has grown in use, said Bonnie Martens, marketing communications specialist at Kalmazoo, Mich.-based Humphrey Products.
"The need for increased flow from smaller valves at lower power is driving the change in the solenoid valve arena," said Martens. "Additionally, the need for quiet valves is also driving these new designs."

Rob Clippard, vice president of sales and marketing at Clippard Instrument Laboratory, Cincinnati, added that Clippard innovated the internal workings of these innovative pneumatic valve designs. "Clippard is the benchmark for valve technology used in medical devices. Our spider design or flat armature spring element provides a valve that is quiet, fast, reliable, and has exceptional life," he said.
The need for higher flows and lower power consumption was what drove Clippard to redesign its benchmark valve design, coming up with its new DV valve, which offers flows up to 100 L/min and only consumes 1.9W in power. Additionally, Clippard said that the company also offers power saving circuits and latching valves in applications where low power use is critical.
Adaptation is critical
Pneumatics is used heavily in oxygen concentrators, infusion pumps, ventilators, wound therapy, blood analyzers, pressure cuff devices, medical bed surfaces, breathable gas delivery systems and anesthesia devices because they all use some type of gas to control their functions.
But because pneumatic components were initially designed for industrial use, manufacturers have had to adapt their use to medical designs. "The challenge is making an industrial-grade product work in a medical device because their needs are different than industrial applications," said Paul Gant, sales manager at Aventics, the newly branded company that was formerly Bosch Rexroth's pneumatics division, based in Lexington, Ky.
However, this forced adaptation has allowed for some unique innovations because there aren't a great deal of regulations or standards that restrict design, Gant added. Although RoHS, REACH and ISO standards are critical to design, there is no one rule to follow. "If you come up with a pretty good idea, there's not a lot of history or regulations that tie you from using that product," Gant said.
Clippard added that adjustments to standard products are application specific. "For example, increasing pressure on the blood pressure monitoring cuffs now would allow you to market your product as a tourniquet," Clippard said, concluding that most medical device manufacturers are looking for partners that can help them simplify their design and manufacturing process.
Custom applications
In the majority of cases, the relationship between pneumatics companies and medical device manufacturers becomes an engineering partnership.

For example, Humphrey was recently recognized for its work with Stryker Medical. Stryker was honored with a 2014 Medical Design Excellence Award through the Medical Device and Diagnostic Industry for its newest hospital bed technology—the Isolibrium critical-care air support surface. The goal of the device is to serve as a tool for nurses to care for patients while the pressure redistribution system helps prevent pressure ulcers in patients. Humphrey was recognized as a "supplier to a winner" for its efforts in the design of the pneumatic controls portion of the Isolibrium Surface.
In another application, Humphrey's custom valve assembly enabled a respiratory care manufacturer to provide critical pressure monitoring when delivering a CO2-O2 mixture to patients suffering from toxic gas or smoke inhalation.
Gant said Aventics' key involvement is with oxygen concentrators. Meeting the low-pressure needs of portable oxygen concentrators (POCs) was a challenge, he said. These portable designs were reduced from 10 lb to about 2 or 3 lb, so adapting an industrial valve to this smaller, lighter weight design required some product redesigns. In industrial settings, pilot-operated valves operate at about 40 psi to open a larger valve. But these medical devices were operating at pressures in the range of 3 to 7 psi.
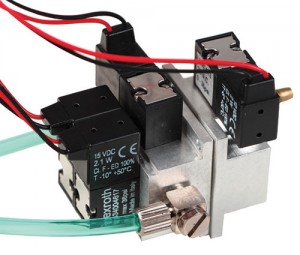
"One of our challenges was to reduce the operating pilot pressure and still use a dual-stage valve, which gives you high flow, but have it operating at less than 6 or 7 psi," Gant said. "To accomplish this, we used polycarbonate bodies that we designed to maximize the area of the operating valve."
As he explained, the physics of fluid power is simple—the pressure to open something is based on the size of what you're pushing. In most valves, the actual operating element is tiny compared to the valve body. "What we needed to do was make the body smaller and the operating element a larger portion of that whole percentage," Gant concluded.
Getting into the Internet of Things
Automation and diagnostics have become a critical part of medical devices, and increasingly, pneumatic components are supporting this need for data transmission, said Gant.
"What this means for pneumatics, is that if the machine is going to get more automated, the pneumatic devices change slightly to where something that might have been a manual device—a toggle valve or something like that—now suddenly has to be electronic and guided by a PLC or motherboard," Gant described, saying that things like pressure control must be electronic and changeable depending on what the device is."
"Theoretically, the more information they have, the more they can tailor your treatment to you. It gives you the best care, but that also means, perhaps, less procedures and less hospital care because more things can be done through the better diagnostics," Gant concluded. "All of these things lead to better personalized healthcare."
![]()
Original Article, October 2014
By Mary Gannon





